We are made from borrowed air.
It stands to reason that since we humans are not green and we don’t photosynthesis everything that constitutes our physical being has come from the consumption of matter that we call food, we have no other way of growing, changing or repairing our physical bodies..
Whether we choose to eat only plants, only animals or both plants and animals, at the end of the food chain is something that lives by photosynthesis, the biological process that uses the energy of light to convert air and water into a substance that can be considered in biological terms to be stored energy.
The chemical equation for this process is 6CO2 + 6H2O → C6H12O6 + 6O2. This means that the reactants, six carbon dioxide molecules and six water molecules, are converted by light energy captured by chlorophyll (implied by the arrow) into a sugar molecule and six oxygen molecules, the products.
Basis of Food Chains
In terrestrial and aquatic ecosystems, photosynthetic organisms, known as primary producers, form the base of the food chain. These include a vast array of plants, phytoplankton, and some bacteria.
Herbivores and Omnivores: These organisms are eaten by herbivores (plant-eaters), which in turn may be consumed by carnivores (meat-eaters) and omnivores (which eat both plants and animals). Each step up the food chain depends on the energy and organic material synthesised by photosynthetic organisms.
As energy is transferred from primary producers up through each trophic level, it becomes less available due to energy being lost as heat and used in metabolic processes. Thus, all higher levels are anchored to the energy captured by photosynthesis.
So what us our food actually made of?
Diagrammatic representation of 95% of plant matter originating from gaseous constituents of the air that surrounds us, converted by biological activity and sunlight into compounds which make their way up through the food chain.
The most basic part of the human food chain is typically primary producers, also known as autotrophs, typically plants.
The majority of a plant's dry weight—around 95%—is derived from the carbon and oxygen that plants obtain from the air. This process occurs primarily through photosynthesis, where plants absorb carbon dioxide (CO2) from the atmosphere and use it to build glucose and other organic molecules. During photosynthesis, oxygen is also released as a byproduct into the atmosphere. It is estimated that about 45% of the dry weight of plants consists of carbon, sourced from CO2.
This substantial contribution from the air includes not only carbon but also oxygen, which gets incorporated into carbohydrates, fats, proteins, and other organic molecules.
The remaining 5% of a plant's dry weight comes from minerals and other nutrients absorbed from the soil. These include nitrogen—obtained through a more complex process known as nitrogen fixation—where soil bacteria convert atmospheric nitrogen into forms that plants can assimilate to synthesise amino acids, the building blocks of proteins.
When animals consume plants, they ingest these amino acids, which are then broken down and reassembled into more complex protein structures as one ascends through the trophic levels. This process underscores the essential role of plants in converting atmospheric components into biological nutrients, which support not only themselves but also the broader food chain.
Nitrogen Fixation: The Key Process
Atmospheric nitrogen, which makes up about 78% of the air, is mostly inert N2 gas that most plants cannot directly utilise. The conversion of this nitrogen into a form usable by plants is achieved through nitrogen fixation. This transformation is critical as it turns nitrogen into ammonia (NH3), a form that plants can assimilate to synthesise amino acids and other nitrogenous compounds.
Biological nitrogen fixation is primarily carried out by symbiotic bacteria known as diazotrophs. These bacteria possess a special enzyme called nitrogenase, which can break the strong bonds of N2, allowing for the incorporation of nitrogen into organic compounds.
In symbiotic relationships, the most well-known nitrogen-fixing bacteria are the rhizobia, which form associations with leguminous plants such as peas, beans, and clover. These bacteria inhabit nodules on the plant roots, where they convert atmospheric nitrogen to ammonia, which the host plant then uses. In exchange, the plant supplies carbohydrates and other organic compounds to the bacteria, derived from photosynthesis.
Some other bacteria and blue-green algae (cyanobacteria) can fix nitrogen independently of plants. These organisms often live in soil or water and can provide nitrogenous compounds to non-leguminous plants indirectly through their activities in the environment.
Assimilation into Amino Acids
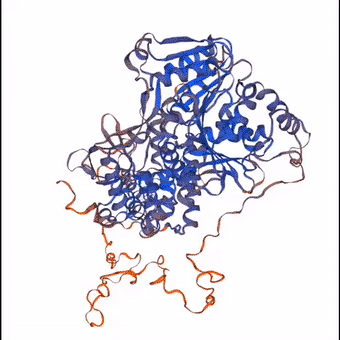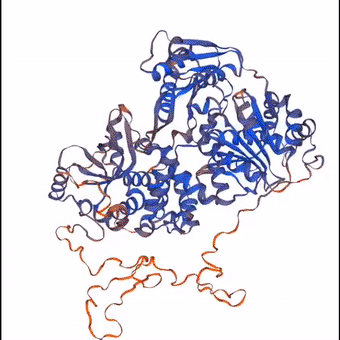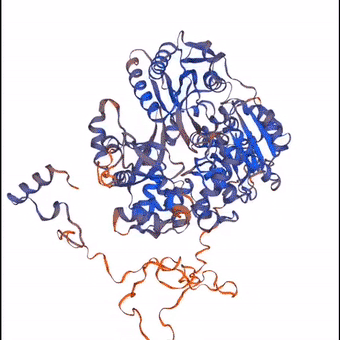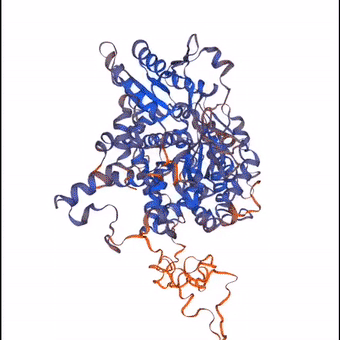
3D structure of a protein molecule made largely from the elements that constitute the air.
Once ammonia is produced via nitrogen fixation, it must be assimilated into amino acids through various pathways:
The Glutamine Synthetase-Glutamate Synthase Pathway involves ammonia first being incorporated into glutamate to form glutamine. Glutamine then reacts to form new glutamate molecules, incorporating more nitrogen.
The Aspartate Aminotransferase Pathway involves the transfer of an amino group to aspartate to form other amino acids.
Once synthesised, amino acids are linked together in various sequences to form proteins, which are crucial for virtually all plant processes, including growth, enzyme production, and responses to environmental stresses.
So if we’re eating plants or animals only 95% of the nutrients we consume come from the air. We are nearly completely made from borrowed air!
The concept that the majority of our nutritional intake—specifically the substance of the plants we consume—is derived from the gases in the air rather than the earth itself invokes profound philosophical reflections on the interconnectedness of life and the seemingly invisible processes that sustain it.
So what about the 5% of our food that doesn’t come from the air?
A consistent 5% loss of nutrients over time would accumulate and quickly deplete resources if they are not replenished, so, before we delve into how these essential nutrients are recycled, let's first identify what they are. These nutrients are generally classified into two categories: macronutrients, which are required in larger quantities, and micronutrients, which are needed in smaller amounts, just as in human nutrition we have macronutrients being fats, carbohydrates and proteins plants too have nutritional requirements.
Macronutrients
Nitrogen (N): Essential for the synthesis of amino acids, proteins, and nucleic acids, nitrogen is a key component of chlorophyll, the molecule that gives plants their green colour and plays a critical role in photosynthesis. This nitrogen although pulled from the soil is fixed there by bacteria and originates in the atmosphere as detailed above,.
Phosphorus (P): Vital for energy transfer within the plant, phosphorus is a part of ATP (the energy currency of cells) and is important for root development and the growth of reproductive structures.
Potassium (K): This nutrient is crucial for the regulation of water and nutrient movement in plant cells, enzyme activation, and overall plant health.
Calcium (Ca): Important for cellular structure and function, calcium also plays a role in soil pH balance and helps stabilise cell walls.
Magnesium (Mg): A central component of chlorophyll, magnesium is necessary for photosynthesis and enzyme activation.
Sulfur (S): Integral to protein synthesis, sulfur is also a part of some vitamins and is important for certain enzyme functions.
Micronutrients
Iron (Fe): Essential for the synthesis of chlorophyll and a crucial component of many enzymes that facilitate oxygen and electron transport.
Boron (B): Important for cell wall formation and reproductive growth, including seed and fruit development.
Zinc (Zn): Plays a significant role in protein synthesis, growth regulation, and development of the reproductive organs.
Copper (Cu): Necessary for photosynthesis and as a component of proteins and enzymes involved in lignin synthesis and metabolism.
Manganese (Mn): Involved in photosynthesis, enzyme activation, and synthesis of various metabolites.
Molybdenum (Mo): Vital for nitrogen fixation in legumes and in the reduction of nitrates within the plant.
Chlorine (Cl): Essential for osmosis and ionic balance, it also plays a role in photosynthesis.
Silicon (Si) and Cobalt (Co) are considered beneficial elements. Silicon can enhance plant strength and resistance to pests and diseases, while cobalt is important for nitrogen fixation in legumes.
So if plants are using these nutrients from the soil how are they replaced and where did they come from in the first place?
Weathering and biological action on bedrock - Most of the soil nutrients originally come from the parent material, which is the underlying geological material (primarily rocks) from which the soil is formed. As these rocks weather chemically and physically, they release minerals into the soil. The initial development of soil involves the accumulation of organic material from dead and decaying plant and animal matter, which gradually enriches the soil as it breaks down and this builds up over time
Volcanic activity - Volcanic activity has been a significant source of rare minerals and nutrients in the soil, enriching it through the emission of ash, lava, and other materials during eruptions. These volcanic ejecta, rich in minerals such as phosphorus, potassium, calcium, magnesium, and trace elements like iron and copper, spread over wide areas and gradually break down to release nutrients into the soil. This process can create highly fertile soils known as andisols, particularly in volcanic regions.
The minerals from volcanic ash contribute to soil fertility over a long period, enhancing plant growth and ecosystem productivity. Additionally, volcanic rocks like basalt, which are abundant in iron and magnesium, weather relatively quickly and further add to the nutrient content of the soil.
The extent to which volcanic materials can circulate globally depends largely on the eruption's magnitude and prevailing atmospheric conditions. Large eruptions can propel ash and gases high into the atmosphere, where they can be transported around the globe by high-altitude winds. This global dispersion can significantly alter atmospheric chemistry and affect surface processes far from the volcano.
Finer materials such as volcanic ash can also travel thousands of kilometers from the eruption site if carried by strong winds, as observed with ash from the 1991 eruption of Mount Pinatubo in the Philippines, which circled the globe within weeks. Moreover, volcanic materials can be washed into oceans and carried by currents to distant ecosystems.
Once deposited, these minerals enter local biogeochemical cycles and become part of the nutrient pool, influencing plant growth and the food chain in various ecosystems. Thus, volcanic activity historically plays a crucial role in contributing rare minerals to soil, significantly impacting both local and global ecosystems.
Oceanic Sources: For coastal areas, seaweeds and other marine organisms can contribute organic matter and nutrients to the soil, once in a growing system these nutrients are transported by forming parts of plants, then animals who are able to move, excrete and decompose away from the coast.
So how are these nutrients replaced?
The recycling of nutrients in soil is essential for ecological sustainability and agricultural productivity. This recycling occurs through a combination of natural processes and human-managed activities that help maintain soil fertility.
Natural Processes:
Decomposition is a key process where bacteria, fungi, and other microorganisms break down the organic matter from dead plants and animals. This releases nutrients back into the soil, allowing them to be reused by other plants. Similarly, the waste products from herbivores, rich in nitrogen, phosphorus, and other nutrients, decompose and enrich the soil, fostering new plant growth. Plants also contribute to this cycle through root exudates, which can enhance microbial activity that aids in nutrient solubilization and uptake. Moreover, leguminous plants have symbiotic relationships with nitrogen-fixing bacteria, transforming atmospheric nitrogen into usable forms for plants, which enriches the soil when these plants decompose.
Human-Managed Recycling Practices:
Practices such as crop rotation help manage soil fertility by alternating nitrogen-fixing legumes with nitrogen-demanding crops, naturally replenishing nitrogen levels. Composting of organic waste like food scraps and garden waste produces a rich soil amendment, adding both nutrients and organic matter back into the soil. Applying organic mulches helps conserve soil moisture and also contributes nutrients as it decomposes. Reduced tillage and no-till farming practices preserve soil structure and microbial life, maintaining the integrity of the soil. Additionally, planting cover crops during off-seasons prevents soil erosion, suppresses weeds, and improves soil structure, further enriching the soil with organic matter when these crops are turned back into the soil. Responsible fertilization based on soil tests can prevent nutrient overloading and reduce pollution risks.
The Ethereal Origin of Life’s Substance
At its core, the fact that plants derive up to 95% of their dry weight from the carbon and oxygen harvested from air via photosynthesis speaks to a fundamental truth about the ethereal, almost intangible nature of life's building blocks. This transformation of invisible gases into solid matter—into the very food we consume—is a testament to the intricate and delicate balance of nature. It highlights a paradox where life's physical substance is largely composed of what we cannot see.
This realisation deepens our understanding of the cycles that bind the terrestrial and the atmospheric, the seen and unseen worlds. As humans, we partake in this grand cycle, consuming the plants that have transformed the air into sustenance, and in turn, exhaling carbon dioxide—fueling the cycle anew. This cyclical exchange underscores a broader ecological reciprocity where every breath is both a biological necessity and a participation in a planetary process.
The fact that our bodies are built from molecules that have cycled through countless forms over eons—from ancient atmospheres to medieval forests to the food on our plates—reflects the transient yet perpetual nature of existence itself. Matter and energy are neither created nor destroyed but eternally reshaped, a concept echoing philosophical and spiritual reflections on the impermanence and continuity of life. Our very essence is not solely of the earth but also of the air; we are as much a part of the sky as we are of the land.
Recognising that the air—a commons that transcends borders and sustains life—is integral to our nourishment also invites ethical considerations about how we treat the environment. Just as deforestation or pollution can degrade the quality of the air, and by extension, the quality of our food, our stewardship of this shared resource becomes a moral imperative. The philosophy of environmental ethics is thus deeply woven into our everyday acts of eating and breathing, urging a respect for the processes that are not only ecological but also profoundly existential.
Finally, the role of air in our nutrition is a scientific truth that illustrates a deeper philosophical unity among diverse life forms. In consuming plants, we are reminded of our shared reliance on a few fundamental elements—carbon, oxygen, and nitrogen. This unity invites a broader reflection on our place within the biosphere, challenging us to see ourselves not as separate from, but as integral to, the web of life that encompasses and connects us all.
In these ways, the seemingly simple act of eating is laden with deeper meanings and connections, bridging the gap between the mundane and the profound, the material and the spiritual, and ultimately, the human and the non-human.
We are made from air and we will return.